As we’ve witnessed during the COVID-19 outbreak, pandemic management at the global and community levels is crucial. The first step to understanding the pervasiveness of an outbreak is through efficient testing and data collection. Serology testing (also known as antibody testing) can identify individuals that have been infected and have developed immunity against a pathogen.[1] This leads to a better understanding of disease pervasiveness and lasting immunity in order to plan and manage the outbreak.
What is a serology test?
A serology test is an antibody test that uses a blood sample to identify signs of a previous infection. It is not intended to diagnose acute or recent cases of infection. It detects antibodies, which are proteins in the blood that help fight off disease.[2] The presence of antibodies may indicate immunity against the pathogen. Humans have three main types of antibodies: immunoglobulin M (IgM), immunoglobulin G (IgG), and immunoglobulin A (IgA).[3] IgA is responsible for the immune response on mucosal surfaces. IgM is one of the first antibodies produced during infection. And IgG typically appears at later stages of infection. COVID-19 serology tests quantify these types of antibodies, which indicate type of transmission and timing of infection.
How is blood collected for a serology test?
Typically, to collect blood for a serology test, a healthcare provider or phlebotomist obtains a blood sample through venipuncture or finger prick. Venipuncture is a very common technique and requires the healthcare provider to find a protruding vein and insert a needle into the vein to draw blood into a vial. During finger prick, an individual or healthcare provider punctures a finger with a lancet, then several drops of blood are collected onto a test strip or in a small capillary collection tube. These procedures are fairly invasive and can be painful.
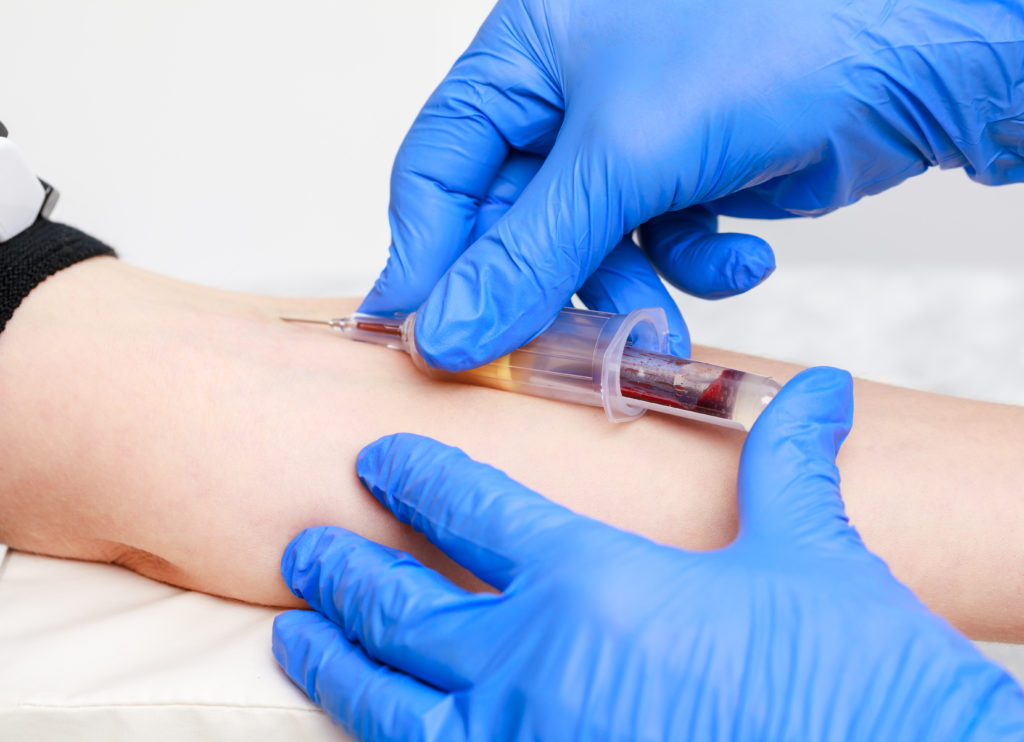
A new blood draw method for serology testing
Traditional methods of blood collection are often painful, time-consuming and inconvenient. The OneDraw™ Blood Collection Device by Drawbridge Health is a viable alternative.
The OneDraw Blood Collection Device, the enabling technology for the FDA-cleared OneDraw™ A1C Test System, is a small, single-use device that draws, collects, and stabilizes a capillary blood sample from the upper arm. Instead of using a traditional hypodermic needle to puncture a patient’s vein, the device is placed on the skin and blood is gently collected using tiny lancets with a light vacuum suction. The blood sample is then stabilized and contained within a removable cartridge, designed to protect the sample during transport. The sample can be sent via regular mail to a designated certified clinical laboratory for the quantitative measurement of HbA1c for monitoring the long-term control of blood sugar (glucose) in people with diabetes.
The OneDraw Blood Collection Device has the potential to be used in the home setting, which has huge implications for current and future pandemic management, particularly containment and minimizing potential exposure to and spread of infection.
Serology testing during COVID-19 and future pandemics
Serology testing for COVID-19 holds a lot of promise, as test results can quantify rates of infection and provide an indication of the pervasiveness of the virus. Large-scale testing, or seroprevalence studies, are being employed to identify symptomatic and asymptomatic individuals who have been infected with COVID-19 and have thus developed antibodies against it.[4] This information gives scientists a better understanding about how the virus is spreading through a population over a certain amount of time.[5] Testing also gives scientists the ability to identify fully-recovered individuals who can become potential convalescent plasma donors, which is a possible treatment for COVID-19.[6] Finally, serology testing has the potential to aid in the diagnosis of patients who initially tested negative with a diagnostic test, but later present with symptoms during the disease course.[7]
More knowledge around seroprevalence means more informed decision making around policy and future preparedness. The current pandemic highlights the necessity for widespread testing and improved methods of blood sample collection to aid in large scale serology testing. Furthermore, better methods for sample collection, such as the OneDraw Blood Collection Device, may enable more testing and has the potential to minimize the risk of exposure and disease spread.
References:
- 1. “Serology (Antibody) Testing,” https://www.uclahealth.org/antibody-serology-testing
- 2. “How Antibodies Work,” https://www.livestrong.com/article/19098-antibodies-work/
- 3. “Human Antibodies,” https://asm.org/Articles/2020/May/COVID-19-Serology-Testing-Explained
- 4. “CDC Large-Scale Seroprevalence Studies,” https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/geographic-seroprevalence-surveys.html
- 5. “CDC Community-level Seroprevalence Studies,” https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/community-level-seroprevalence-surveys.html
- 6. “Convalescent Plasma Therapy,” https://www.mayoclinic.org/tests-procedures/convalescent-plasma-therapy/about/pac-20486440
- 7. “COVID-19 Testing, PCR versus Serology,” https://www.health.nd.gov/sites/www/files/documents/Files/MSS/coronavirus/COVID19_PCRvsSerologyTesting.pdf